Identifying the Neural Link Between Gut Bacteria and Social Behavior in Mice
Could the germs that live inside of our bodies be affecting our ability to socialize and make friends? Research conducted in recent decades suggests that the answer—for mice—is yes.
Research has shown that the communities of bacteria that live in a mouse's gut are essential for the animals to exhibit normal social behavior with other mice. Mice that have been bred to be germ-free, without a gut microbiome, display significant antisocial behaviors, such as avoiding a stranger mouse rather than interacting with it. How do microbes influence an animal's behavior? In other words, what is the chain of events happening on the molecular and cellular levels, from gut bacteria to the brain to behavioral changes?
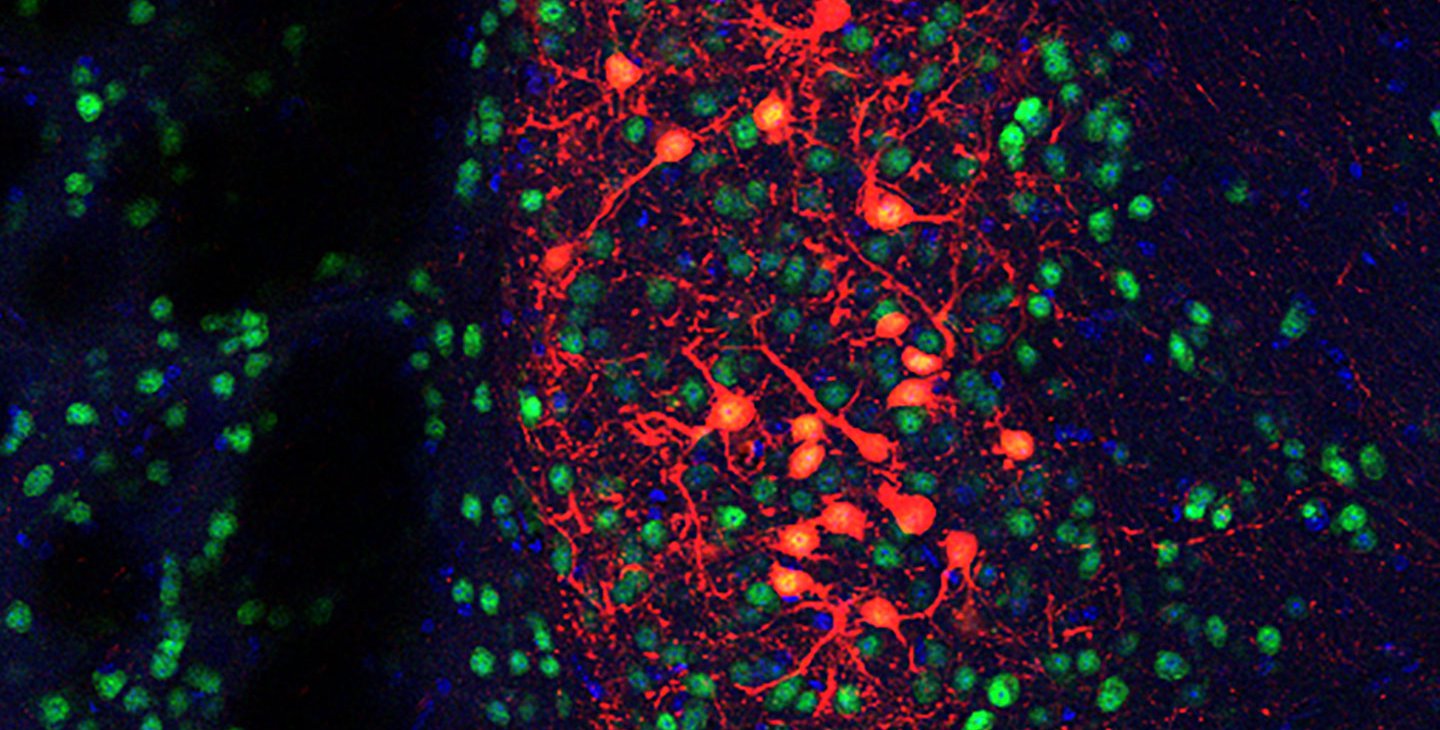
Now, a new study has identified a specific circuit of neurons that is directly influenced by the gut microbiome and is subsequently responsible for antisocial behaviors in mice that lack a gut microbiome. Transplants of fecal matter from mice with healthy gut microbiomes into these germ-free mice were sufficient to change the activity of these neurons and thus improve their social behavior. The researchers also identified a specific bacterial species that can increase sociability.
Identifying the interactions between gut microbes, neurons, and whole-organism health effects (like behavioral changes) may be an important line of inquiry into ways to, someday, help improve social deficits, such as those concurrent with depression and autism. Current practices for treating these types of issues include prescribing pharmaceuticals, such as antidepressants and anxiolytic drugs. However, it is difficult to get these drugs into the right brain regions at the right concentrations, and much of the drug ends up throughout the body. Understanding the gut–brain connections adds to evidence that neuropsychiatric disorders can be indirectly improved by treating the gut microbiome, which is much easier to access pharmaceutically than the brain.
The research was conducted primarily in the laboratory of Sarkis Mazmanian, Luis B. and Nelly Soux Professor of Microbiology and Heritage Medical Research Institute Investigator. A paper describing the study appears in the journal Nature on June 30.
It had already been shown that, on a chemical level, germ-free mice have significantly higher levels of the hormone corticosterone (the analog of the so-called stress hormone, cortisol, in humans) than mice with healthy microbiomes. The team of researchers, led by former Mazmanian lab postdoctoral scholar Wei-Li Wu, aimed to identify the neurons that were both affected by corticosterone and played a role in social behaviors.
"By altering the mouse's microbiome, we were able to change levels of corticosterone: less microbiome means more stress hormone," says Mazmanian. "There are a lot of neurons in the body that respond to corticosterone—called glucocorticoid receptor-positive neurons—and we wanted to know which cell populations and brain regions were then responsible for the altered social behaviors in germ-free mice?"
After identifying several subsets of neurons in the brain involving stress control, the team used chemical and genetic tools to artificially block corticosterone from activating these neurons in mice without a microbiome. These mice, despite lacking a gut microbiome, were able to exhibit more normal social behavior because their neurons were not responding to the stress hormone.
So, what was it about the gut bacteria—or lack thereof—that was causing increased corticosterone levels in the first place? To address this, the team conducted fecal transplants from wild-type mice with normal gut microbiota into germ-free mice. These mice then showed decreased corticosterone levels and more normal social behavior. The team then systematically identified a species of bacteria that mediated this improvement, namely Enterococcus faecalis. Germ-free mice that were colonized with E. faecalis showed improved social behaviors and lowered corticosterone levels. The mechanisms through which E. faecalis is able to mediate this improvement will be the subject of future research.
"A number of studies have shown that the gut microbiome impacts complex behaviors in mice, such as sociability. The underlying neuronal circuits that mediate the microbiome's influence on behavior had not been previously discovered. This work strengthens the emerging appreciation of the profound effects of the gut-brain connection," says Mazmanian. "Conceptually, the findings lay the groundwork for exploring similar effects in humans."
Wu is now a faculty member at National Cheng Kung University in Taiwan. Future directions for this research include zooming in closer to the host-bacteria relationship, identifying the molecular signals produced by gut microbes, and how these signals influence the host. Mazmanian and Wu plan to continue their collaboration.
"Our study benefited tremendously from a number of collaborations, including critical contributions from the laboratories of Viviana Gradinaru and Rustem Ismagilov at Caltech," says Mazmanian.
The paper is titled "Microbiota regulate social behavior via stress response neurons in the brain." Wei-Li Wu is the study's first author. In addition to Mazmanian, Caltech co-authors are former research technician assistant Mark Adame; graduate student Jacob Barlow; former Mazmanian lab postdoctoral scholar Gil Sharon; former graduate student Catherine Schretter (PhD '19); postdoctoral scholar Brittany Needham, former undergraduate Madelyn Wang (BS '19); graduate students Weiyi Tang, James Ousey, and Reem Abdel-Haq; former research associate Keith Beadle; Viviana Gradinaru (BS '05), professor of neuroscience and biological engineering, Heritage Medical Research Institute Investigator, and director of the Center for Molecular and Cellular Neuroscience at the T&C Chen Institute for Neuroscience; and Rustem Ismagilov, Ethel Wilson Bowles and Robert Bowles Professor of Chemistry and Chemical Engineering, and director of the Jacobs Institute for Molecular Engineering for Medicine. Additional co-authors are Chia-Wei Liou, Tzu-Ting Lai, Yuan-Yuan Lin, and Tzu-Hsuan Yao of National Cheng Kung University in Taiwan. Funding was provided by the Ministry of Science and Technology in Taiwan, Higher Education Sprout Project, Ministry of Education to the Headquarters of University Advancement; a National Institutes of Health (NIH) Biotechnology Leadership Pre-doctoral Training Program (BLP) Fellowship; the National Science Foundation Graduate Research Fellowship Program; the Jacobs Institute for Molecular Engineering for Medicine, the Kenneth Rainin Foundation Innovator Award; and Lynda and Blaine Fetter, Charlie Trimble (BS '63, MS '64), the Heritage Medical Research Institute, and the NIH. Sarkis Mazmanian is an affiliated faculty member with the Tianqiao and Chrissy Chen Institute for Neuroscience at Caltech.
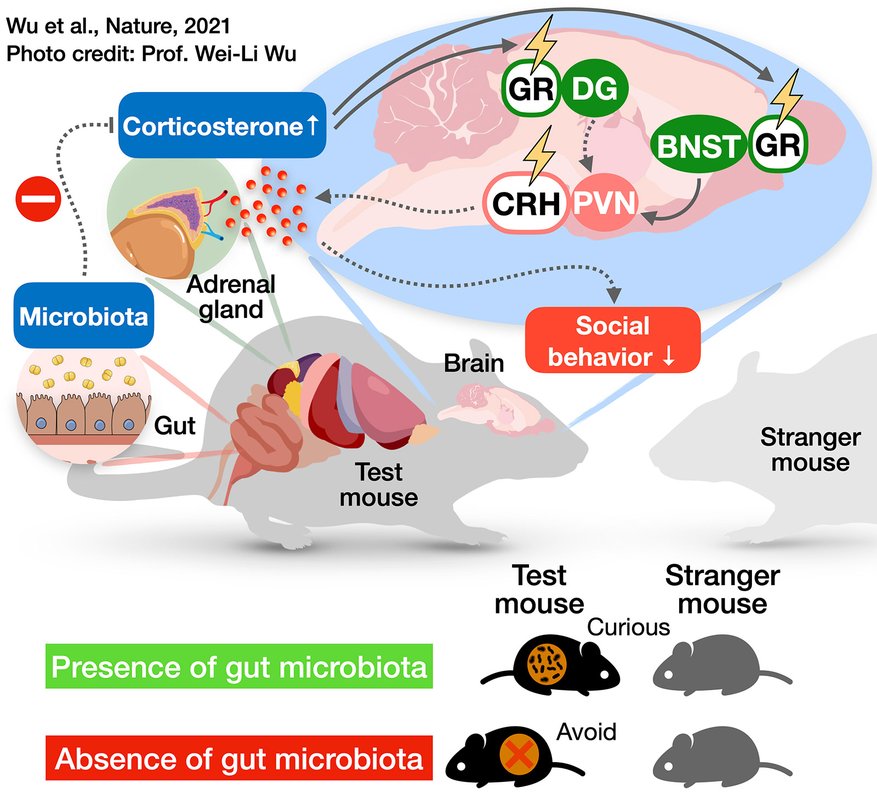
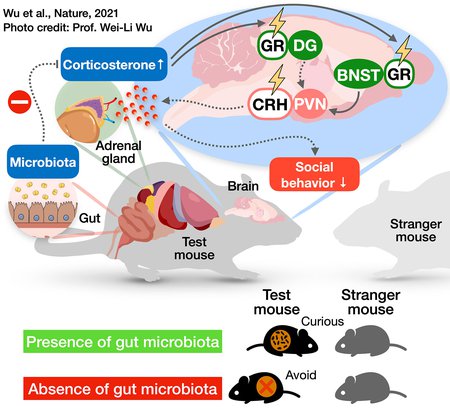 This diagram illustrates the cascade of changes occurring in the mouse body and brain in the absence of a gut microbiome. Lack of gut microbes leads the adrenal gland to produce more corticosterone, a stress hormone, which then influences a neural circuit controlling social behavior in the brain, making the mouse exhibit antisocial behaviors.
Credit: Wei-Li Wu (Caltech & NCKU)
This diagram illustrates the cascade of changes occurring in the mouse body and brain in the absence of a gut microbiome. Lack of gut microbes leads the adrenal gland to produce more corticosterone, a stress hormone, which then influences a neural circuit controlling social behavior in the brain, making the mouse exhibit antisocial behaviors.
Credit: Wei-Li Wu (Caltech & NCKU)